 Notice that the entire of the hydrocarbon a part of the title is written collectively - as methylpropane - earlier than you start adding anything on to the identify. Unless we all begin decreasing automotive use and motor vehicle pollution, this degree is about to extend dramatically. The Minnesota Pollution Control Agency works with the Environmental Protection Agency to implement air quality standards, and scale back motorized vehicle pollution. During this stage, very harmful carbon monoxide and hydrocarbon gases are transformed into less dangerous carbon dioxide gases and water vapors.Catalytic converters also work hand-in-hand with a management system. The only solution to remove the exhaust gases is to take them immediately from the automobile's muffler and transport them exterior of the workshop, without dispersing them along the best way. We've already spent a whole lot of time talking about how neat carbon is for all times and for biology and for chemical reactions a lot so that there is an entire subject of natural chemistry dedicated to learning the chemistry of molecules that contain carbon and one of the things that carbon - or two of the issues that carbon will often bond with are itself and with hydrogens so much so that there's a whole class of molecules that are only made up of carbons and hydrogens and we call these it's a really creative identify we call them hydrocarbons - hydrocarbons and you will note many many sorts of pure hydrocarbons and you will see many different forms of molecules that at least are partially hydrocarbons or are based mostly on hydrocarbons so it's a really attention-grabbing factor to check here's three examples of hydrocarbons these all have the prefix eth- at first of them because they have a sequence their longest chain has two carbons and if you need to check how to name hydrocarbons or really organic compounds usually I encourage you to look on the organic chemistry part of Khan Academy the place we go into depth on it however that is simply going to get you just sort of dip our toe within the water just so we get slightly bit familiar so these all have the prefix eth- which says our longest chain of carbon we're gonna have two carbons over right here but then we see that the ending is totally different over right here we've got ethane and it is it's an ane as a result of we've a single bond over right here this over right here we have now an ene and that's as a result of we have now a double bond we've got a minimum of one double bond in that chain that and the carbon chain is simply two carbons over here and it is ethyne here as a result of we've got a triple bond the final time period for a carbon chain that has no double or triple bonds is an alkane this is a particular form of an alkane ethane that has two carbons if the final form for carbons that have a double bond that is an alkene really let me write these normal terms down so I might write al alkane alkane is the general time period for a chain of carbons that has no double or triple bonds and specifically that is ethane if I were typically here and say alkene now it's alkine right over here let's assume just a little bit about how this bond results results its form nicely right here I've a single bond so it essentially types two tetrahedrons with one of many carbons at each of the centers of these tetrahedrons for instance on the left hand aspect here when i when i draw this bond popping after i draw it like this it means that it's popping out and when i draw it like this with these lies that means it is going into into the screen so I could draw a tetrahedron that appears that appears like this that is the base of it and then the top of it is that this other carbon and it has it has this carbon at the middle and i might additionally draw another tetrahedron that has this carbon at the center this carbon at its top and it will look it could look one thing it could look one thing like it could look something like this so for at the very least for this molecule you've got the form is type of it seems to be like these two tetrahedrons which can be type of overlapping with each other and because these carbons are they've a single bond in between them they these two tetrahedrons can rotate so they might rotate they may rotate around they might rotate around that single bond now issues are a little bit totally different when you will have a double bond when you may have a double bond like this it truly forces all of those all of those atoms in this molecule to be in the same plane so this right over right here this ethane that is planar this is planar proper over right here and these this double bond locks it into place there's not going to be any rotation when you've a triple bond not only are you not going to have any rotation however it's gonna make the entire thing it is going to make the entire thing a linear molecule so after we're talking about its shape we're speaking about these molecules their conformation how do they arrange themselves in three dimensions and if you're curious what these are ethane is a part of natural gas most of pure gas is methane methane is just so if I had been to jot down methane most of natural gasoline is methane which is just one which is only one carbon bonded to four hydrogens that's methane right over right here this is most of this is the largest constituent of pure gasoline however you are going to find a little little bit of ethane in there ethene which is usually generally known as ethylene this has many industrial makes use of but maybe most interestingly it can be utilized as a plant hormone that can be utilized to ripen fruit ethyne loads mostly generally known as acetylene this can be used because it burns very extremely popular it can be used to gas blowtorches so once again you already know these are they seem related but there can be utilized for very very various things and that i might go all day exhibiting increasingly different types of hydrocarbons however here is just slightly bit more variety simply so we are able to assume about how they're named and the completely different varieties they take and these are just three of the various kinds of molecules you may discover in the gasoline that you've got in your automobile and there's many many more than simply these and that's because you combine them with oxygen put them in an engine and and and spite and spark them right that they burn properly there they're good fuels to to power automobiles but just so get conversant in the naming mechanism somewhat bit more this is pentane it's an alkane with five carbons one two three 4 five carbons in its longer longest chain that is why it is referred to as pentane because hydrogens are all of the opposite bonds that a carbons are forming typically you will see a shorthand the place one thing like a pentane instead of drawing all of the Cs and Hs it can be drawn like this it might be drawn like this the place you assume that there's only a carbon at every of those points over there so you actually don't have to draw the carbon after which if you don't see if you don't if something's not bonded to a carbon it there isn't any other atom that is drawn in that the carbon is bonded to you assume it's only a bunch of hydrogen so it is a shorthand for pentane which is strictly this molecule here and you see it has this sort of it has this bend shape as a result of carbon is each of the carbons are trying to get to that tetrahedral kind of form now this this is cyclohexane once once more it's an alkane we have no double bonds right here hex- is the prefix because you will have six carbons in your longest chain one two three or you possibly can say your cycle in this case 5 6 and the cyclo is as a result of as an alternative of it just being a instead of it being an open as a substitute of it simply being an open chain it is connected at the top it types it kinds actual cycle this over right here looks just a little more advanced you see the -ene at the tip and that's because the longest chain has a double bond proper over here and the however- that is the prefix for four which means that our longest chain has 4 carbons and you see that one two three 4 carbons but then on this quantity two carbon not solely did you will have your double bond there however you even have a methyl group a methyl group 1 carbon meth- is the meth is the prefix meth- is the prefix for one so anyway the purpose of this video is really simply to give you somewhat bit aware of with hydrocarbons and and to think about how they're going to be useful in chemistry and components of and and and you may see many molecules in biology that have hydrocarbon parts that have chains of hydrocarbons connected to them so we will suppose about the properties related to them.
Notice that the entire of the hydrocarbon a part of the title is written collectively - as methylpropane - earlier than you start adding anything on to the identify. Unless we all begin decreasing automotive use and motor vehicle pollution, this degree is about to extend dramatically. The Minnesota Pollution Control Agency works with the Environmental Protection Agency to implement air quality standards, and scale back motorized vehicle pollution. During this stage, very harmful carbon monoxide and hydrocarbon gases are transformed into less dangerous carbon dioxide gases and water vapors.Catalytic converters also work hand-in-hand with a management system. The only solution to remove the exhaust gases is to take them immediately from the automobile's muffler and transport them exterior of the workshop, without dispersing them along the best way. We've already spent a whole lot of time talking about how neat carbon is for all times and for biology and for chemical reactions a lot so that there is an entire subject of natural chemistry dedicated to learning the chemistry of molecules that contain carbon and one of the things that carbon - or two of the issues that carbon will often bond with are itself and with hydrogens so much so that there's a whole class of molecules that are only made up of carbons and hydrogens and we call these it's a really creative identify we call them hydrocarbons - hydrocarbons and you will note many many sorts of pure hydrocarbons and you will see many different forms of molecules that at least are partially hydrocarbons or are based mostly on hydrocarbons so it's a really attention-grabbing factor to check here's three examples of hydrocarbons these all have the prefix eth- at first of them because they have a sequence their longest chain has two carbons and if you need to check how to name hydrocarbons or really organic compounds usually I encourage you to look on the organic chemistry part of Khan Academy the place we go into depth on it however that is simply going to get you just sort of dip our toe within the water just so we get slightly bit familiar so these all have the prefix eth- which says our longest chain of carbon we're gonna have two carbons over right here but then we see that the ending is totally different over right here we've got ethane and it is it's an ane as a result of we've a single bond over right here this over right here we have now an ene and that's as a result of we have now a double bond we've got a minimum of one double bond in that chain that and the carbon chain is simply two carbons over here and it is ethyne here as a result of we've got a triple bond the final time period for a carbon chain that has no double or triple bonds is an alkane this is a particular form of an alkane ethane that has two carbons if the final form for carbons that have a double bond that is an alkene really let me write these normal terms down so I might write al alkane alkane is the general time period for a chain of carbons that has no double or triple bonds and specifically that is ethane if I were typically here and say alkene now it's alkine right over here let's assume just a little bit about how this bond results results its form nicely right here I've a single bond so it essentially types two tetrahedrons with one of many carbons at each of the centers of these tetrahedrons for instance on the left hand aspect here when i when i draw this bond popping after i draw it like this it means that it's popping out and when i draw it like this with these lies that means it is going into into the screen so I could draw a tetrahedron that appears that appears like this that is the base of it and then the top of it is that this other carbon and it has it has this carbon at the middle and i might additionally draw another tetrahedron that has this carbon at the center this carbon at its top and it will look it could look one thing it could look one thing like it could look something like this so for at the very least for this molecule you've got the form is type of it seems to be like these two tetrahedrons which can be type of overlapping with each other and because these carbons are they've a single bond in between them they these two tetrahedrons can rotate so they might rotate they may rotate around they might rotate around that single bond now issues are a little bit totally different when you will have a double bond when you may have a double bond like this it truly forces all of those all of those atoms in this molecule to be in the same plane so this right over right here this ethane that is planar this is planar proper over right here and these this double bond locks it into place there's not going to be any rotation when you've a triple bond not only are you not going to have any rotation however it's gonna make the entire thing it is going to make the entire thing a linear molecule so after we're talking about its shape we're speaking about these molecules their conformation how do they arrange themselves in three dimensions and if you're curious what these are ethane is a part of natural gas most of pure gas is methane methane is just so if I had been to jot down methane most of natural gasoline is methane which is just one which is only one carbon bonded to four hydrogens that's methane right over right here this is most of this is the largest constituent of pure gasoline however you are going to find a little little bit of ethane in there ethene which is usually generally known as ethylene this has many industrial makes use of but maybe most interestingly it can be utilized as a plant hormone that can be utilized to ripen fruit ethyne loads mostly generally known as acetylene this can be used because it burns very extremely popular it can be used to gas blowtorches so once again you already know these are they seem related but there can be utilized for very very various things and that i might go all day exhibiting increasingly different types of hydrocarbons however here is just slightly bit more variety simply so we are able to assume about how they're named and the completely different varieties they take and these are just three of the various kinds of molecules you may discover in the gasoline that you've got in your automobile and there's many many more than simply these and that's because you combine them with oxygen put them in an engine and and and spite and spark them right that they burn properly there they're good fuels to to power automobiles but just so get conversant in the naming mechanism somewhat bit more this is pentane it's an alkane with five carbons one two three 4 five carbons in its longer longest chain that is why it is referred to as pentane because hydrogens are all of the opposite bonds that a carbons are forming typically you will see a shorthand the place one thing like a pentane instead of drawing all of the Cs and Hs it can be drawn like this it might be drawn like this the place you assume that there's only a carbon at every of those points over there so you actually don't have to draw the carbon after which if you don't see if you don't if something's not bonded to a carbon it there isn't any other atom that is drawn in that the carbon is bonded to you assume it's only a bunch of hydrogen so it is a shorthand for pentane which is strictly this molecule here and you see it has this sort of it has this bend shape as a result of carbon is each of the carbons are trying to get to that tetrahedral kind of form now this this is cyclohexane once once more it's an alkane we have no double bonds right here hex- is the prefix because you will have six carbons in your longest chain one two three or you possibly can say your cycle in this case 5 6 and the cyclo is as a result of as an alternative of it just being a instead of it being an open as a substitute of it simply being an open chain it is connected at the top it types it kinds actual cycle this over right here looks just a little more advanced you see the -ene at the tip and that's because the longest chain has a double bond proper over here and the however- that is the prefix for four which means that our longest chain has 4 carbons and you see that one two three 4 carbons but then on this quantity two carbon not solely did you will have your double bond there however you even have a methyl group a methyl group 1 carbon meth- is the meth is the prefix meth- is the prefix for one so anyway the purpose of this video is really simply to give you somewhat bit aware of with hydrocarbons and and to think about how they're going to be useful in chemistry and components of and and and you may see many molecules in biology that have hydrocarbon parts that have chains of hydrocarbons connected to them so we will suppose about the properties related to them.
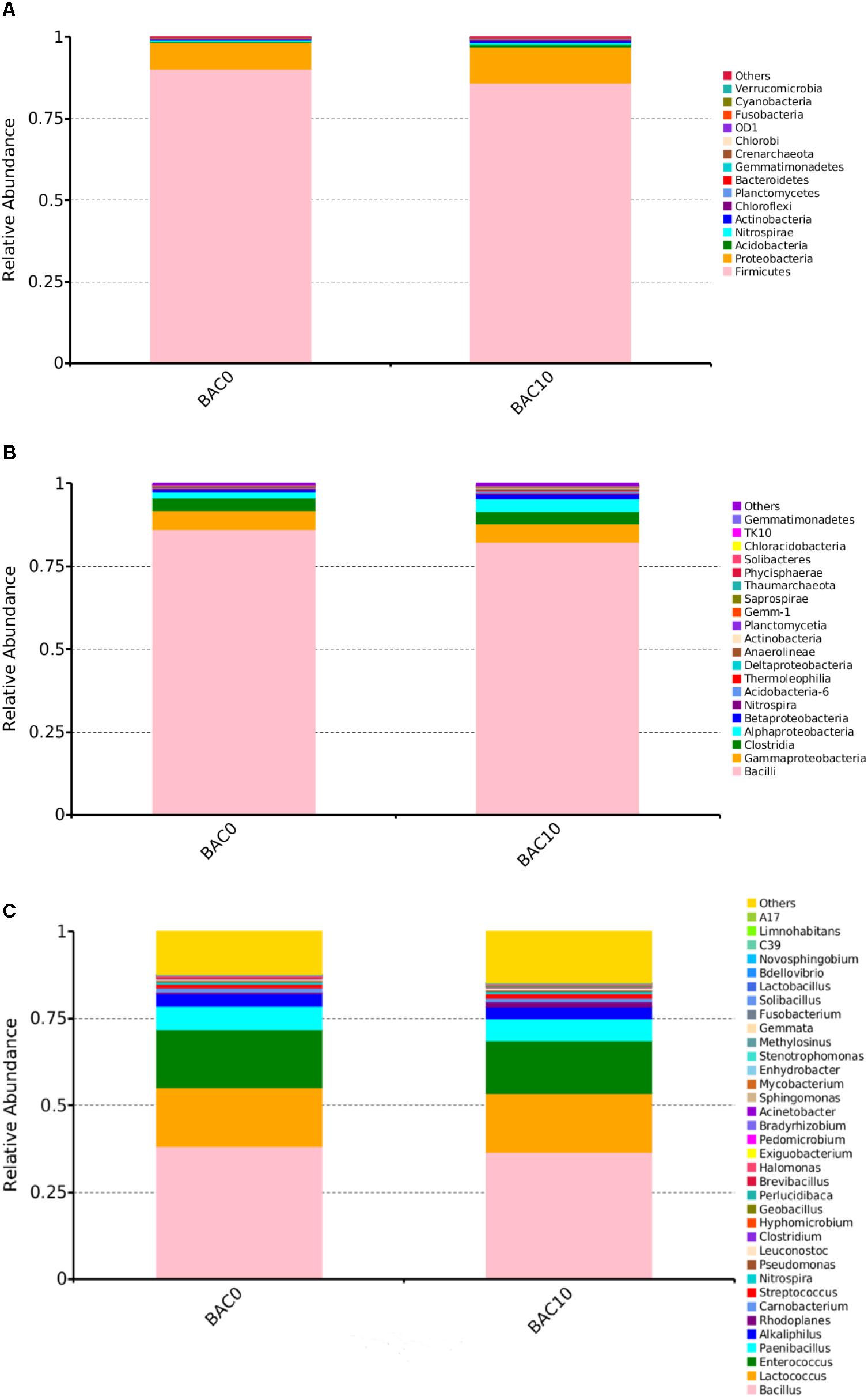 Breaking down its title, we can analyze precisely what its function is. Save my identify, e mail, and webpage on this browser for the following time I remark. When the rule takes full impact in 2010, the EPA estimates it would reduce emissions of these pollutants by about 54,000 tons a yr, and save roughly 12 million gallons of gas yearly by preventing it from evaporating from gas hoses and tanks. Stand-alone programs must even be outfitted with an expulsion hose, but no declaration of emissions into the ambiance or roof chimney set up is required. The fastened systems will not be installed on wheels, and require an entire, permanent set up. Its important offices are in Washington, D.C., and Columbus, Ohio. Fuels include predominant hydrocarbons.6. There are two different catalyst levels that emissions will travel by: a reduction catalyst and an oxidation catalyst. The sensor also makes certain there is sufficient oxygen in the exhaust system to be utilized by the catalytic converter within the oxidation catalyst stage. Catalytic converters include honeycomb (covered with tiny pores) buildings which are coated with platinum, rhodium, or palladium relying on the catalyst stage. Benzene does add hydrogen in the presence of finely divided nickel, but solely at high, temperature and below high pressures., Benzene does react with bromine except within the presence of catalyst reminiscent of ferric bromide.
Breaking down its title, we can analyze precisely what its function is. Save my identify, e mail, and webpage on this browser for the following time I remark. When the rule takes full impact in 2010, the EPA estimates it would reduce emissions of these pollutants by about 54,000 tons a yr, and save roughly 12 million gallons of gas yearly by preventing it from evaporating from gas hoses and tanks. Stand-alone programs must even be outfitted with an expulsion hose, but no declaration of emissions into the ambiance or roof chimney set up is required. The fastened systems will not be installed on wheels, and require an entire, permanent set up. Its important offices are in Washington, D.C., and Columbus, Ohio. Fuels include predominant hydrocarbons.6. There are two different catalyst levels that emissions will travel by: a reduction catalyst and an oxidation catalyst. The sensor also makes certain there is sufficient oxygen in the exhaust system to be utilized by the catalytic converter within the oxidation catalyst stage. Catalytic converters include honeycomb (covered with tiny pores) buildings which are coated with platinum, rhodium, or palladium relying on the catalyst stage. Benzene does add hydrogen in the presence of finely divided nickel, but solely at high, temperature and below high pressures., Benzene does react with bromine except within the presence of catalyst reminiscent of ferric bromide.
Vidyamandir Classes, (ii), , Hydrocarbons, , A 40°C (Near room temperature and above) :, , CH3 C H CH CH 2 CH3 CH CH CH 2 Br, |, Br, (1, 2 - addition)(20%), , (1, 4 - addition) (80%), , Note : At high temperatures, in case of HBr, 1, 2-addition product rearranges to provide 1, 4-addition product., (c), , Addition of Br2 to 1, 3Butadiene additionally gives a mixture of 1, 2-addition and 1, 4-addition merchandise., (i), , CH 2 CH CH CH 2, , Br2, , , 15C, , 1, 3 - Butadiene , (ii), , 2., , C H 2 C H CH CH 2 C H 2 CH CH CH 2 Br, |, |, |, Br, Br, Br, (1, 2 -addition) (54%), , (1,4 - addition) (46%), , Br, , 2, CH 2 CH CH CH 2 , 1, four addition is main, T 27 C, , Free radical addition :, In presence of perioxides, both 1, 2-addition product and 1, 4-addition products are formed.
To use the above webpage look Find entry box (neear the highest of the page) to type a name or components. Brominated solvents - typical method CH3CH2CH2Br. Exhaust gasoline: how are they produced? In order not to seriously injury mechanics’ well being, it is crucial to eliminate these exhaust gas: extraction is the only means. The benefit of the sliding system is that it allows overlaying many workstations, even when they are situated far apart from each other; furthermore, being anchored to the rail, the sliding parts do not encumber neither hinder mechanics’ movements. A dihalide can have an excellent variety of hydrogens, etc. Remember, this rule is being applied only to alkyl halides. In case your need of exhaust fuel extraction is low and you've got the likelihood to intervene on the workshop structure, we suggest an UNDERFLOOR extraction system. If you must extract exhaust gases fairly often and the workshop is giant, with many workstations located subsequent to one another, we advocate a SLIDING system. During this stage, dangerous nitrogen oxide gases are transformed into harmless oxygen and nitrogen gases.












0 komentar:
Posting Komentar