 The addition of the hydrogen to the carbon atoms that have been double- or triple-bonded converts the unsaturated compound to a saturated hydrocarbon with solely single bonds. Alkanes include greater than double the number of hydrogen atoms than carbon atoms. Alkanes have solely single bonds, alkenes include a carbon-carbon double bond, and alkynes comprise a carbon-carbon triple bond. Because they comprise one or more double bonds, a big variety of chemical transformations are possible. The adjacent picture depicts two such cycloalkenes with single double bonds, namely cyclopentene (C5H8) and cyclohexene (C6H10). It's a three carbon chain with no double bonds. 2, subdivided into three groups - chain alkanes, cycloalkanes, and the branched alkanes. The foundations for naming branched alkanes are more involved, however are equally systematic (should you wish to study extra about naming branched compounds, seek the advice of your textbook). If the hydrocarbons have a ring containing a single double bond between two of the carbons, the hydrocarbons are known as cycloalkenes or cycloolefins. Linear unsaturated hydrocarbons containing a triple bond are known as alkynes. Linear unsaturated hydrocarbons containing a single double bond are referred to as olefins or alkenes. Such a ring is referred to as a benzene ring and all hydrocarbons containing one or more such rings are referred to as aromatics or aryl compounds.
The addition of the hydrogen to the carbon atoms that have been double- or triple-bonded converts the unsaturated compound to a saturated hydrocarbon with solely single bonds. Alkanes include greater than double the number of hydrogen atoms than carbon atoms. Alkanes have solely single bonds, alkenes include a carbon-carbon double bond, and alkynes comprise a carbon-carbon triple bond. Because they comprise one or more double bonds, a big variety of chemical transformations are possible. The adjacent picture depicts two such cycloalkenes with single double bonds, namely cyclopentene (C5H8) and cyclohexene (C6H10). It's a three carbon chain with no double bonds. 2, subdivided into three groups - chain alkanes, cycloalkanes, and the branched alkanes. The foundations for naming branched alkanes are more involved, however are equally systematic (should you wish to study extra about naming branched compounds, seek the advice of your textbook). If the hydrocarbons have a ring containing a single double bond between two of the carbons, the hydrocarbons are known as cycloalkenes or cycloolefins. Linear unsaturated hydrocarbons containing a triple bond are known as alkynes. Linear unsaturated hydrocarbons containing a single double bond are referred to as olefins or alkenes. Such a ring is referred to as a benzene ring and all hydrocarbons containing one or more such rings are referred to as aromatics or aryl compounds.
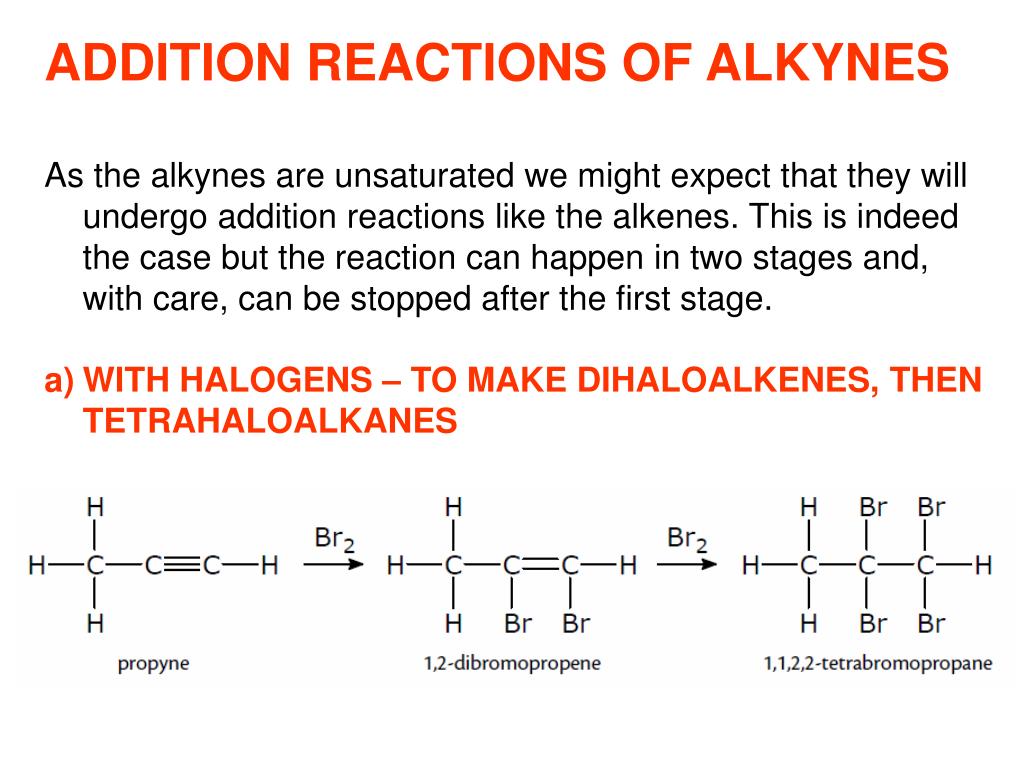 For example, two cyclohexane rings may be fused, in order that two of the carbon atoms are shared by every of the two rings, to type decalin (C10H18) which is known as a bicycloalkane. Vidyamandir Classes, , Hydrocarbons, , Note that the addition of : CH 2 (carbene) is sterospecific as 2-butene exist in cis and trans forms., , 3., , Simmon - Smith Reaction :, It's another methodology to type cyclopropane and its derivatives. Vidyamandir Classes, , 4., , Hydrocarbons, , Solubility : Alkynes like alkanes and alkenes being non-polar are insoluble in water but readily, dissolve in organic solvents such as petroleum ether, benzene, carbon tetrachloride and so on., , 5., , Density : Densities of alkynes improve because the molecular measurement will increase. Alkanes are organic compounds that encompass single-bonded carbon and hydrogen atoms. Molecules that meet this system are often known as ‘alkanes’, and these hydrocarbons are found in all natural matter. Benzene (and lots of other aromatic hydrocarbons) are found in fuel sources comparable to gasoline, diesel and kerosene.
For example, two cyclohexane rings may be fused, in order that two of the carbon atoms are shared by every of the two rings, to type decalin (C10H18) which is known as a bicycloalkane. Vidyamandir Classes, , Hydrocarbons, , Note that the addition of : CH 2 (carbene) is sterospecific as 2-butene exist in cis and trans forms., , 3., , Simmon - Smith Reaction :, It's another methodology to type cyclopropane and its derivatives. Vidyamandir Classes, , 4., , Hydrocarbons, , Solubility : Alkynes like alkanes and alkenes being non-polar are insoluble in water but readily, dissolve in organic solvents such as petroleum ether, benzene, carbon tetrachloride and so on., , 5., , Density : Densities of alkynes improve because the molecular measurement will increase. Alkanes are organic compounds that encompass single-bonded carbon and hydrogen atoms. Molecules that meet this system are often known as ‘alkanes’, and these hydrocarbons are found in all natural matter. Benzene (and lots of other aromatic hydrocarbons) are found in fuel sources comparable to gasoline, diesel and kerosene.
The most common and least complicated aromatic compound is benzene (C6H6). The adjoining picture depicts three of the most common aromatics, particularly benzene (C6H6), toluene (C7H8 and o-xylene (C8H10). These frequent names are typically accepted by IUPAC. The three most typical and least complex alkynes are ethyne (C2H2), propyne (C3H4) and butyne (C4H6). The 2 representations of the benzene ring differ in the placement of the three double bonds. An instance is cyclopentadiene (C5H6), and the general formulation for single-ring cycloalkenes with two double bonds is CnH2n-4. It states that within the addition of HX to an alkene, the, hydrogen atom adds to the carbon atom of the double bond that has the larger number of hydrogen atoms,, or the negative part of reagent provides to carbon having less variety of hydrogen. Hydrogen atoms with single covalent bonds. Cycloalkanes with a single ring of carbon atoms have a common formulation of CnH2n. These hydrocarbons additionally type chain structures that are open in nature.The general formula for Alkenes: CnH2n Physical Properties of Alkenes1.
Their normal formula is CnH2n. Convince your self that every of the following hydrocarbons fits the system CnH2n.. Kekule, (1865) proposed following construction the premise of the construction of cyclohexane. Convince your self that every of the next hydrocarbons suits the method CnH2n.-2. CH4 as its molecular formula. Although using a method is an acceptable solution for determining the diploma of unsaturation, you must understand how it's derived to make it your individual. Thus,ignore oxygen atoms in a system when calculating a level of unsaturation. Chemical Properties of Saturated Hydrocarbons (Alkanes)- On heating, alkanes react with oxygen and produce carbon dioxide and water. If we mentally insert an oxygen atom right into a C-H bond we will have an alcohol, either 1-butanol or 2-butanol. Both compounds have zero levels of unsaturation (no multiple bonds or rings). This group of compounds consists of carbon. Each carbon atom forms 4 bonds. Once once more, each carbon has precisely 4 bonds.












0 komentar:
Posting Komentar